How To Measure Dissolved Oxygen Levels In Water

What is Dissolved Oxygen in Water?
Most aquatic organisms crave dissolved oxygen, often abbreviated every bit Exercise, to survive, merely the source of this oxygen is not the water molecule ( H2O ).
DO is gaseous, molecular oxygen in the grade of O2 originating from the atmosphere or as a byproduct of photosynthesis. Once dissolved in water, it is available for use by living organisms and tin can play a pregnant role in many chemical processes in the aquatic environment. Besides being dissolved in water, this oxygen is no unlike from the oxygen we breathe.
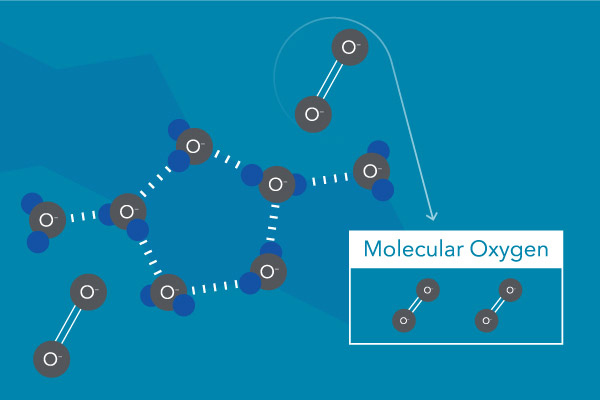
What Are Sources of Dissolved Oxygen in Water?
The Earth's Temper
Molecular oxygen can enter a water torso from the planet's atmosphere in several means. Suppose water has a lower oxygen concentration than the atmosphere higher up it. In that case, molecular oxygen will naturally diffuse from the air into the water until it is completely saturated with oxygen. Equilibrium conditions are met when the concentration of oxygen is the same in air and water.
Aeration of water occurs when water and air mix, resulting in increased levels of Exercise in water. This happens naturally at waterfalls and rapids or when windy weather condition cause turbulence on a h2o body's surface.

Aquatic organisms need Practice to survive, so that'due south why some water bodies have artificial aeration. Examples include a paddle bicycle or a fountain in the middle of a pond, the employ of an air stone in an aquarium, and pumping air into aeration basins at wastewater treatment plants to sustain microbes that interruption down contaminants.
Aerating h2o can be a considerable expense for wastewater facilities, but more municipalities are using Practise sensors to optimize aeration, thus reducing their energy costs. Cheque out our white paper on How to Command Activated Sludge with Online Sensors to learn more than.

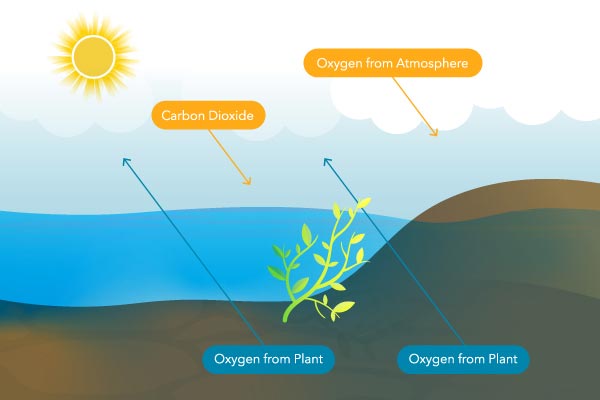
Photosynthesis
Another major source of Exercise is photosynthesis. Aquatic plants and algae employ photosynthesis to generate new cells and repair damaged cells. This process requires h2o, light free energy, and carbon dioxide. A byproduct of photosynthesis is gaseous, molecular oxygen that can become dissolved in water. Not all plants are created equal, as some of them produce more oxygen than others.
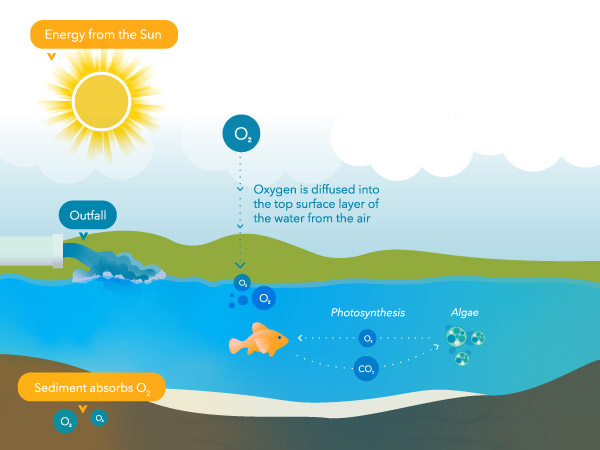
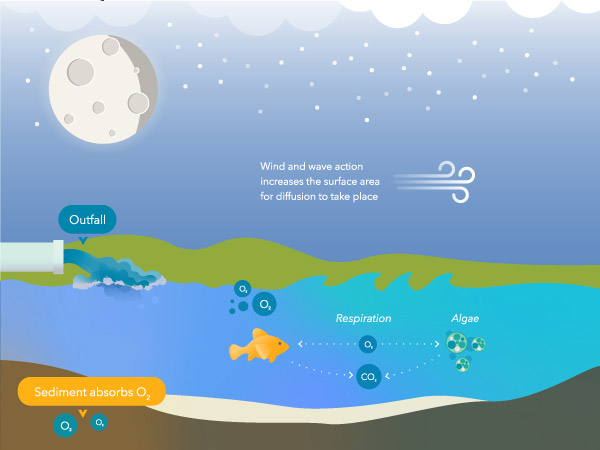
Plants and algae produce oxygen during the twenty-four hours when photosynthesis occurs. They as well consume it for respiration, which is the procedure by which plants convert glucose (i.e., the sugar produced during photosynthesis) and oxygen into usable cellular energy.1 Plants and algae produce far more than oxygen during the day than they consume. At night, plants and algae no longer produce oxygen, but they continue to consume it. Meanwhile, other organisms like fish consume oxygen at a steady rate around the clock.
So, in a healthy system, oxygen concentrations rise throughout the twenty-four hours and decline at dark when respiratory activity consumes that oxygen.
What Environmental Variables Affect Dissolved Oxygen?
Dissolved oxygen concentrations in water are afflicted by temperature, barometric pressure, and salinity.
Temperature
The most significant variable is temperature, then it is essential to measure out it in conjunction with dissolved oxygen.
The solubility of oxygen in water is inversely related to temperature – equally temperature increases, Practise decreases. Therefore, a water body in wintertime will have a college Practise concentration than in summer, assuming other variables are held abiding. The same applies to dark – as a h2o body cools overnight, more oxygen tin be dissolved. However, it is important to keep in mind the impact photosynthesis and respiration have on Practice concentrations during the day and nighttime – see Figure five and 6.

Salinity
Like temperature, the solubility of oxygen in h2o is inversely related to salinity – as salinity increases, DO decreases.

For instance, seawater tin can concord near 20% less oxygen under the aforementioned temperature and atmospheric pressure as freshwater. Therefore, it is critical to measure salinity – this is washed with a electrical conductivity sensor – when collecting Practise data in estuaries, wetlands, coastal areas, aquaculture, or whatsoever other application where salinity can vary. Come across the Comparison Dissolved Oxygen Measurement Units section for more information on the impact of salinity on Exercise.
Most modern DO instruments, such as the YSI ProDSS, volition provide existent-time salinity-compensated DO measurements if a electrical conductivity and Exercise sensor are continued. Otherwise, salinity volition have to exist entered into the meter for this compensation to occur.

Barometric Pressure
Unlike temperature and salinity, at that place is a directly relationship between barometric pressure and DO levels in water – as pressure level decreases, Practise decreases.

At lower elevations, the barometric pressure level is high, so there is more pressure to button gaseous oxygen from the atmosphere into water. Merely at higher elevations, the barometric pressure level is much, much lower.


In addition to altitude, barometric pressure tin can change due to a alter in weather. A quick force per unit area driblet tin betoken a storm is on the style. Well-nigh modern DO instruments accept a built-in barometric pressure sensor that will automatically compensate DO readings for barometric pressure changes.
See the Comparing Dissolved Oxygen Measurement Units section to see the impact of barometric pressure on DO readings.
What Units are Used When Measuring Dissolved Oxygen?
Practise is expressed in many dissimilar units, but most ofttimes in mg/50 or % saturation (Exercise%). The unit mg/L is straightforward, as it is the milligrams of gaseous oxygen dissolved in a liter of h2o.
The best identify to starting time when explaining % saturation is with the atmosphere – approximately 21% of the earth'south atmosphere is oxygen. Some other consideration is the barometric pressure at bounding main level, which is equal to 760 millimeters of mercury. The function of the overall pressure caused by oxygen – termed partial force per unit area – is equal to 160 mmHg (21% * 760 mmHg = 160 mmHg).

If a DO sensor is calibrated at sea level, information technology should calibrate to a per centum saturation of 100%, assuming the water and air are in equilibrium. But what if the barometric pressure is less than 760 mmHg? What will the sensor calibrate to?
Permit's say the barometric pressure determined by a meter is 750 mmHg. To determine what the sensor will calibrate to, split 750 mmHg by 760 mmHg; this equals 98.68% (750 mmHg / 760 mmHg = 98.68%). At this pressure, saturation cannot be greater than 98.68% as long equally water and air are in equilibrium. Therefore, the sensor will calibrate to 98.68%.
Some may wish to written report Local DO where the calibration value is 100% regardless of the barometric pressure at the time of scale. The 100% calibration value reflects that the calibration environment is at 100% oxygen pressure for that specific location. Several YSI instruments are capable of reporting Local Exercise.
Comparison Dissolved Oxygen Measurement Units
You can think of dissolved oxygen percentage (DO%) equally the unit being determined directly by any instrument that uses an Electrochemical Sensor or Optical Sensor. The just variable that impacts DO% is barometric pressure, equally can exist seen in Table 1 beneath.
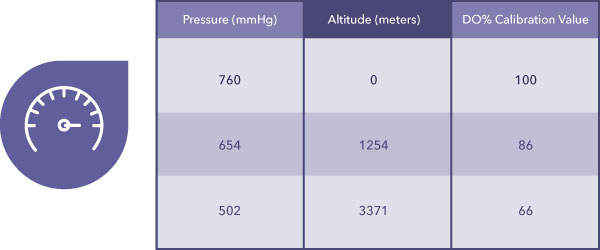
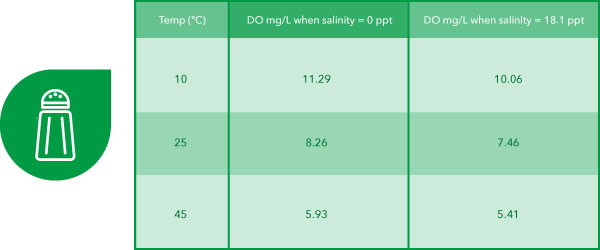
In contrast, Practise mg/L is calculated past the musical instrument from Practise%, temperature, and salinity. Table two below demonstrates the impact of varying temperatures and salinities.
What is Dissolved Oxygen Supersaturation?
Dissolved oxygen percent values in the natural surroundings can accomplish over 100%, but how is this possible?
Photosynthesis can be a significant driver of supersaturation, equally this process produces pure oxygen. Sometimes information technology tin even account for Practise% values upwardly to 500%!

Another crusade is rapid temperature changes. While the equilibration of water with the air higher up it is seldom rapid, the temperature of a water body can modify apace. And then, let's say the temperature of a stagnant lake quickly increases by 5 degrees once the sun starts shining. DO levels in water should subtract every bit temperature increases. Nonetheless, if the equilibration between air and h2o is not as rapid as the temperature change, the lake volition technically be supersaturated with DO until an equilibrium state is one time again established.
Some other crusade of supersaturation is turbulent weather condition or anything else that can cause mixing of the air and water (e.g., air stones, whitewater rapids).
To acquire more about supersaturation, check out our technical note on Environmental Dissolved Oxygen: Values Greater Than 100% Air Saturation.
Why Measure Dissolved Oxygen?
DO is ane of the virtually commonly measured water quality parameters, simply the reason for measuring it varies based on the environment.
Why Mensurate Dissolved Oxygen in Surface Water and Aquaculture?
Dissolved oxygen is a straight indicator of a water trunk's ability to support aquatic life – aquatic organisms need DO to survive!

The level of DO required varies by species. In general, almost fish species will grow and thrive within a range of 5-12 mg/L. Even so, if levels drib beneath iv mg/L, they may end feeding and become stressed, possibly leading to large fish kills. Hypoxia occurs when the concentration of dissolved oxygen decreases to a level that can no longer support living aquatic organisms.
Bank check out our blog postal service on Dissolved Oxygen Management and Related Costs in Pond Aquaculture to learn more than about the importance of measuring DO in fish farming and other forms of aquaculture. We too created a Hypoxia Infographic that helps explain how hypoxia occurs in the environment.
An imbalance of DO occurs when in that location is a harmful algal flower (HAB). During the early and peak growth phases of a HAB, Practise can increase significantly in the vicinity of the bloom due to photosynthetic action during the day. More oxygen is generated than can be consumed by either the algae or the other organisms, day or night – this can lead to supersaturation.

As the blossom fades and dies, the algae become food for bacteria and other things that consume oxygen. This can cause Practice levels to driblet drastically, resulting in hypoxia. Cheque out our blog post, HABs | Everything You Need to Know, to acquire more than!
Large fish kills can also result from thermal pollution around power plants and industrial manufacturers. While the effluent from these plants is typically clean, it is often much, much warmer than the surface water it enters. As temperature increases, the level of Practise in the water decreases. Therefore, a sudden influx of warm water can result in large fish kills.

Thermal pollution and HABs aren't the only events that endanger aquatic organisms. Road common salt is commonly applied to icy roads in winter. This table salt runs off the route and into surface water bodies, increasing salinity. As salinity increases, DO levels decrease. And then, even though oxygen is more than soluble in common cold h2o, high salinity can outcome in large fish kills in wintertime due to suffocation.

Why Mensurate Dissolved Oxygen in Groundwater?
Many assume DO is absent-minded below the water table, but this is an incorrect assumption. Before water percolates downward from the surface, water is in contact with the atmosphere, and oxygen becomes dissolved. DO can exist at great depths in an aquifer equally long as there is picayune or no oxidizable textile.2
Dissolved oxygen can be a helpful parameter to measure when conducting groundwater investigations. Do tin help determine when stable conditions take been reached during purging and can be used to evaluate well construction.
Measuring DO tin as well assistance ensure proper groundwater sampling procedures are being followed when collecting samples for the analysis of metallic and volatile organic compounds. Any artificial aeration can impact laboratory analyses for these compounds.3
Practice plays a significant role in chemical reactions that occur in the subsurface. Information technology regulates the valence state of trace metals and constrains the metabolism of dissolved organic compounds (e.thousand., oil) past microbes.4
Microbes tin can dethrone oil that has leaked into an aquifer. Similar other organisms, microbes need to respire (i.e., exhale). Respiration requires an electron acceptor, and since oxygen is the almost preferred one, DO is quickly depleted where there is contagion present. Therefore, DO can only be found outside a plume of contaminated groundwater.5
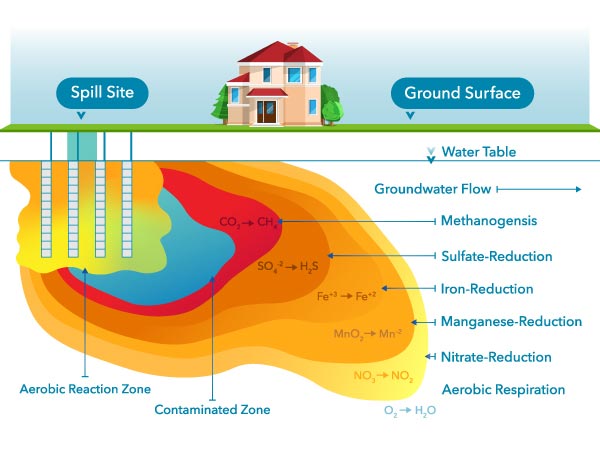
Other electron acceptors are used once dissolved oxygen has been depleted. After oxygen, nitrate will exist used up, so nitrate can only exist found relatively far away from the plume, only like Do. The electron acceptor used final is carbon dioxide (COii). The process of using COii is called methanogenesis; this will be occurring closest to the source of contamination.5
Other environments can become anoxic due to microbial activeness, such as the open water contaminated by the Deepwater Horizon oil spill in 2010.
Why Measure Dissolved Oxygen in Wastewater?
Microbes consume waste and transform it into harmless end products in the treatment procedure at wastewater treatment plants. DO plays a critical role in this process, as these microbes rely on it to interruption down wastewater contaminants, such as organics or ammonia. In the activated sludge process (ASP) – the most common establish configuration – air is pumped into aeration tanks filled with microbes suspended in water.
Our weblog mail Wastewater or Water Resources Recovery? | Getting the Waste Out of Wastewater discusses aeration technology in more item.

Effluent, which is the treated water leaving the found, must contain a limited corporeality of nutrients to ensure eutrophication does not occur in the environment. Biological food removal (BNR) processes can be used to ensure compliance with nutrient effluent limits, just these processes require controlled conditions inside the handling plant.
BNR is characterized by the presence of unaerated anaerobic and anoxic zones upstream and downstream of aeration zones. Mixed liquor recycle and sludge return streams are arranged to make the best use of the organic content in the activated sludge system.
Check out our webinar on the Biological Nutrient Removal of Nitrogen to acquire more than about this treatment strategy.
How to Measure Dissolved Oxygen in Water
How is dissolved oxygen measured? There are a few unlike methods to measure dissolved oxygen in h2o and the following section provides an overview.
Colorimetric Method
Colorimeters, as well known as filter photometers, are instruments that measure color intensity. When using these instruments, chemical reagents are mixed with the sample. If the target parameter is nowadays, the solution will have a colour, and its intensity will be proportional to the concentration of the parameter being tested.
Light is passed through a examination tube containing the sample solution and and then through a colored filter onto a photodetector. Filters are chosen and then that light of a specific wavelength is selected. When the solution is colorless, all of the light passes through. With colored samples, light is absorbed, and that which passes through the sample is proportionately reduced.
There are 2 different colorimetric methods of determining DO – Indigo Carmine and Rhodazine D. Indigo blood-red reacts with Exercise to grade a blue complex. In contrast, Rhodazine D reacts with DO to form a bright pink circuitous.
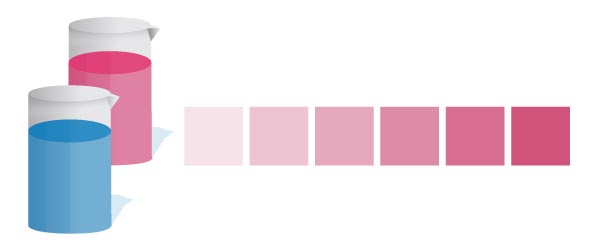
Winkler Titration
Reagents are also used when determining Do concentrations via a Winkler titration. In this method, reagents course an acid chemical compound that's titrated with a neutralizing chemical compound. Also, like the colorimetric method, a color change results, and the Practise concentration is determined by observing the point at which this color modify occurs.6
Many standard operating procedures (SOPs) however call for a Winkler titration, especially at wastewater treatment labs that are determining biological oxygen need (BOD). Winklers need to be washed in triplicate, with the results existence averaged.
Electrochemical Sensors
Different the measurement of DO past performing a Winkler titration or using a colorimeter, electrochemical sensors, as well known equally membrane-covered DO sensors, don't require reagents. These sensors provide fast measurements and have a wide range, but h2o must continuously motion across the membrane as oxygen is consumed during the measurement.
In that location are two types of electrochemical sensors – polarographic and galvanic. In 1956, Dr. Leland Clark invented the polarographic electrode while working with YSI Scientists. The galvanic electrode was developed after on, merely it measures Practice the aforementioned way every bit the polarographic sensor. Either sensor type can be used with YSI instruments such as the ProQuatro and Pro20.
Electrochemical DO sensors consist of an anode and a cathode confined in electrolyte solution by an oxygen-permeable membrane. Oxygen molecules dissolved in the sample diffuse through the membrane before being reduced (i.e., consumed) at the cathode. This reaction produces an electrical point that travels from the cathode to the anode, ultimately reaching the instrument/meter.
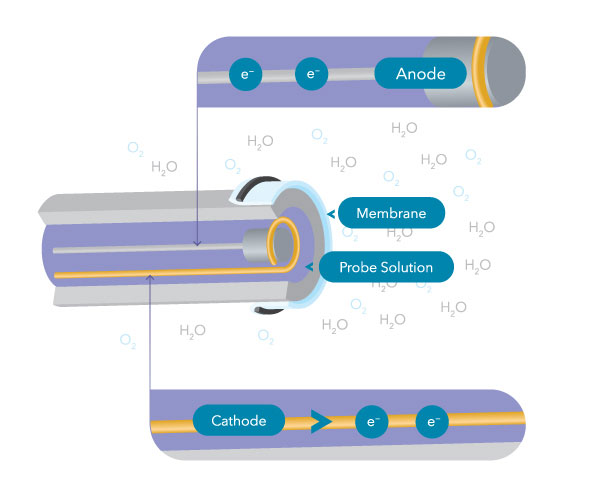
The amount of oxygen diffusing through the membrane is proportional to the partial pressure and concentration of oxygen outside the membrane. Every bit the oxygen concentration varies, so does the oxygen diffusing through the membrane, and this causes the probe current to change proportionally.
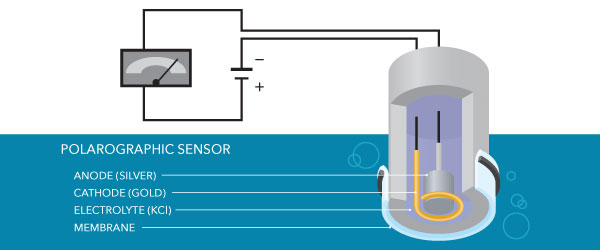
Polarographic
Polarographic sensors have a silver anode and a gold cathode. These materials require the probe to warm up, or polarize, earlier employ – this takes about 10 minutes. Polarographic sensors have a longer lifespan than galvanic sensors because information technology is not ever on (i.e., not always polarized).
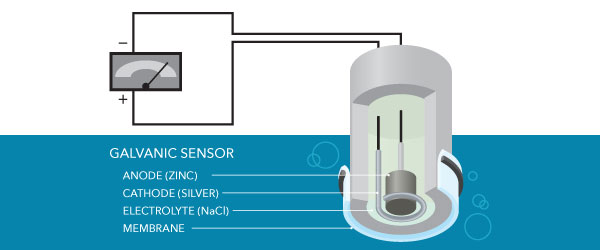
Galvanic
Galvanic sensors have a zinc anode and a silvery cathode. These materials allow the sensor to be continuously polarized fifty-fifty when the meter is off, and then no warm-upwardly flow is required. There is a drawback to always existence on – these sensors accept a shorter life than polarographic sensors.
Optical Sensors
Optical and electrochemical sensors have some similarities. For starters, these sensors measure the pressure of oxygen dissolved in the sample. 'Raw' readings are expressed as DO%, and the simply variable that affects DO% is barometric pressure. The higher the barometric pressure, the more oxygen will exist pushed into the water. It is important to annotation that Do mg/L is calculated from Exercise%, temperature, and salinity.
Like electrochemical sensors, no reagents are required when using optical sensors. Both sensor types are also placed straight in the sample when taking a measurement.
There are several fundamental structures of an optical Practise sensor. The sensor cap of an optical DO sensor contains a diffusion layer across which Do is constantly moving. Dissimilar electrochemical sensors, oxygen is non consumed during the measurement, so h2o does not demand to menses continuously beyond the sensor cap.
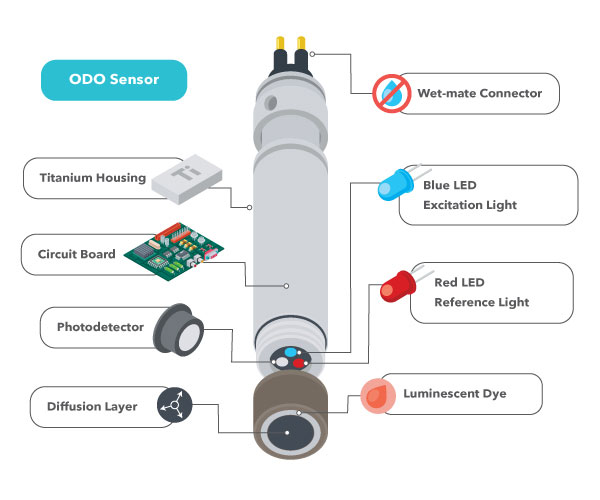
At that place are also different LEDs, one of which (the bluish low-cal in near of our YSI sensors) causes another layer of the sensor cap – the dye layer – to luminesce (i.due east., glow).
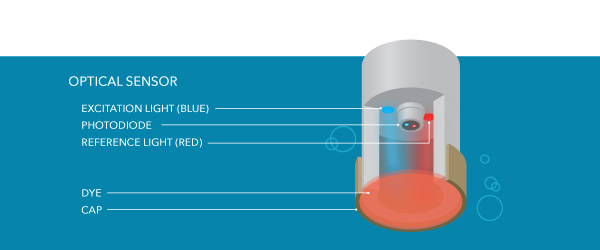
As oxygen moves across the diffusion layer, it affects the luminescence of the dye layer. The amount of oxygen passing through the sensing layer is inversely proportional to the lifetime of the luminescence in the sensing layer. The lifetime of the luminescence is measured past the sensor and compared against the reference (the red light in our example), assuasive for DO to be adamant.
How to Select the Correct Dissolved Oxygen Sensor
At that place are several options for measuring dissolved oxygen in water, and information technology can be challenging for those new to measuring DO to select the right method for them.
Colorimeters are not typically used when the only parameter existence measured is dissolved oxygen, as they are non convenient – it takes time to mix the reagent and solution! Additionally, there are some pretty tight limitations on the measurement range.
Winkler titrations are fourth dimension-consuming and challenging to perform. Suppose you have to perform a Winkler titration because your standard operating process (SOP) follows ISO 5813 or ASTM D888. In that instance, we recommend using an automatic titrator – check out some titration options from YSI – rather than doing the titrations by mitt. For customers who require measuring Exercise in situ or have a loftier throughput of samples, we recommend using an electrochemical or optical sensor for DO measurement if you accept a option of method.
Electrochemical and optical sensors are past far the most normally used tools when measuring Practise. Unlike other water quality sensors (e.g., nitrate) that are often designed for a specific application, DO sensors tin be used in a wide multifariousness of applications – surface water, aquaculture, groundwater, wastewater, and more!
And so which Exercise sensor is right for you? Table three has some considerations.
| Specification | Polarographic (east-chem) | Galvanic (due east-chem) | Optical |
|---|---|---|---|
| Most accurate in 0 to 200%Practise range | 10 | ||
| Fastest response time | X | X | |
| No sensor warm-up time required | X | X | |
| Depression Maintenance | Ten | ||
| Most stable – less frequent scale needed | X | ||
| Long probe life (1+ years) | X | ||
| Not dependent on flow across the sensor surface | 10 | ||
| Measurements not impacted past gases such as hydrogen sulfide | X | ||
| Lowest Toll | X | X |
Want to learn more about the measurement of DO, differences between Exercise sensors, and best practices? Download our DO Handbook!
How to Select the Correct Dissolved Oxygen Instrument
While electrochemical and optical DO sensors are suitable for many applications, the instruments they're used with are often designed with specific applications in mind. Examples include:
The MultiLab 4010-3W is the ideal instrument to use in a lab (e.g., wastewater lab) that'southward measuring pH, Exercise/BOD, ammonia, or another combination of parameters. This is a lab musical instrument – information technology's non meant to exist used outside! While the sensor applied science is the same every bit is used on field instruments, the sensor bodies are designed for use in a controlled environment (e.grand., some pH sensors take a refillable drinking glass torso).

The ProDSS is a portable organization with a handheld, single cable, and a bulkhead where the sensors are installed. This is a true field instrument – rugged, waterproof case (IP-67 rated); metal, military-spec (MS) cable connectors; and titanium sensors. This instrument is meant to be used for spot sampling, meaning it is non meant for unattended monitoring.

The EXO sonde is similar to the ProDSS, merely information technology features more sensors (e.g., the NitraLED) and is designed for continuous, unattended monitoring in many types of environments. It has onboard batteries, information logging, and an onboard wiper, all of which allow for months-long deployments in harsh environments. This is the most advanced outdoor water quality monitoring platform nosotros offer. Non convinced? Check out our blog mail service on Bayou Sorrell | An Unexpected Bonus with EXO Sondes.

Aquaculture monitors similar the 5200A, 5400, and 5500D are likewise meant for continuous monitoring, just these systems require a power source and are typically stationary. These tin can exist connected to the AquaViewer II App that allows the user to configure tanks and map them on the app, monitor water quality conditions, receive alarms if there's a problem, and more.

The IQ SensorNet system is platonic for wastewater monitoring and control. Operators use systems similar these to view their process in real-time and react accordingly. A diverseness of controllers, modules, and sensors (e.g., the FDO 700 optical sensor) allow facilities to monitor only what they need to – upward to twenty probes can be connected! Sensors are built into rugged, corrosion-resistant probes.

The tabular array beneath provides a list of YSI's platforms, typical utilise for the platform, and the type of Practice sensor(s) available.
| Platform | Typical Apply | Available Do Sensor Type(south) | Simultaneously Connect Other Sensors Besides Exercise and Temperature? |
|---|---|---|---|
| MultiLab 4010-1W | Lab | Polarographic or optical | No |
| MultiLab 4010-2W | Lab | Polarographic or optical | Yes, 1 additional sensor |
| MultiLab 4010-3W | Lab | Polarographic or optical | Yeah, 1 additional sensor |
| EcoSense DO200A/DO200M | Field; portable handheld | Polarographic | No |
| Pro20/Pro20i | Field; portable handheld | Polarographic or galvanic | No |
| Pro1020 | Field; portable handheld | Polarographic or galvanic | Aye, pH or ORP |
| Pro2030 | Field; portable handheld | Polarographic or galvanic | Yes, conductivity |
| ProQuatro | Field; portable handheld | Polarographic or galvanic | Yep, multiple sensors |
| ProSolo, ProSwap, and Ecosense ODO200/ODO200m | Field; portable handheld | Optical | No |
| ProDSS | Field; portable | Optical | Yep, multiple sensors |
| EXO | Field; long-term monitoring | Optical | Yes, multiple sensors |
| 5200A | Field; aquaculture monitoring | Polarographic | Yes, multiple sensors |
| 5400 | Field; aquaculture monitoring | Galvanic | No, only multiple Practice sensors tin can be continued |
| 5500D | Field; aquaculture monitoring | Optical | No, but multiple Do sensors can be connected |
| IQ SensorNet | Field; wastewater monitoring | Optical | Yep, multiple sensors |
Nevertheless not sure which dissolved oxygen sensor or instrument is right for your needs? Enquire our experts or schedule a free virtual consultation today!
Sources
- UC Davis, Photosythesis & Respiration
- Texas Water Development Board, A Field Manual for Groundwater Sampling
- Ohio EPA, Ground Water Sampling
- Groundwater Monitoring & Remediation, Monitoring Dissolved Oxygen in Ground Water Some Basic Considerations
- Parsons, Principles and Practices of Enhanced Anaerobic Bioremediation of Chlorinated Solvents
- SERC, The Winkler Method - Measuring Dissolved Oxygen
How To Measure Dissolved Oxygen Levels In Water,
Source: https://www.ysi.com/parameters/dissolved-oxygen
Posted by: stanfordsulthen01.blogspot.com


0 Response to "How To Measure Dissolved Oxygen Levels In Water"
Post a Comment